Hi dear users. I want to define optical properties of CsI(Tl). I don’t understand how determine FASTCOMPONENET and SLOWCOMPONENET array according to energy range.
Thanks for telling me What is the method of determining these components?
For example, the following example is given in the Geant4 manual and I want to know how Scnt_FAST and Scnt_SLOW are obtained for other scintillators according to the energy range.
G4double Scnt_PP[NUMENTRIES] = { 6.6eV, 6.7eV, 6.8eV, 6.9eV,
7.0eV, 7.1eV, 7.2eV, 7.3eV, 7.4eV };
G4double Scnt_FAST[NUMENTRIES] = { 0.000134, 0.004432, 0.053991, 0.241971,
0.398942, 0.000134, 0.004432, 0.053991,
0.241971 };
G4double Scnt_SLOW[NUMENTRIES] = { 0.000010, 0.000020, 0.000030, 0.004000,
0.008000, 0.005000, 0.020000, 0.001000,0.000010 };
G4Material Scnt;
G4MaterialPropertiesTable* Scnt_MPT = new G4MaterialPropertiesTable();
Scnt_MPT->AddProperty(“FASTCOMPONENT”, Scnt_PP, Scnt_FAST, NUMENTRIES);
Scnt_MPT->AddProperty(“SLOWCOMPONENT”, Scnt_PP, Scnt_SLOW, NUMENTRIES);
Hello,
The FASTCOMPONENT and SLOWTCOMPONENT properties in the G4MaterialPropertiesTable (MPT) correspond to the emission spectra for each component on the scintillator. Which are associated with emission spectra for their respective time constants: FASTTIMECONSTANT and SLOWTIMECONSTANT in the MPT.
Geant4 takes the input of energy rather than the wavelength for these properties as if often listed in manufacturers datasheets. The manufacturers of scintillators usually just give the overall emission spectrum for the scintillator. Some manufacturers will give the details regarding emission spectra and time constant information, for example, this Saint-Gobain CsI data sheet gives a fast time constant of 16 ns with a peak emission of 315 nm and a slow component of 1 us with a peak emission of 500 nm. This information would need to be converted into the correct units and put in the source code of your simulation. If the manufacturer does not provide this information you will need to experimentally measure this yourself if you require this information per component. Otherwise, just use the overall emission spectrum and apply it to both the FASTCOMPONENT and SLOWCOMPONENT properties and set the time constants.
I hope this helps.
Thanks Hubbard. Your answer was very helpful
Hi guys . I am in the same situation but in this case I handle the scintillation crystal NaI(Tl) , but I do not understand well how to find the matrix FASTCOMPONENET and SLOWCOMPONENET already having the decay constant which is 230ns with an emission peak at 415nm , I would appreciate your help.
Best regards
Hi Sebastian,
You can use Figure 1 in this catalog
sodium-iodide-material-data-sheet.pdf (174.2 KB)
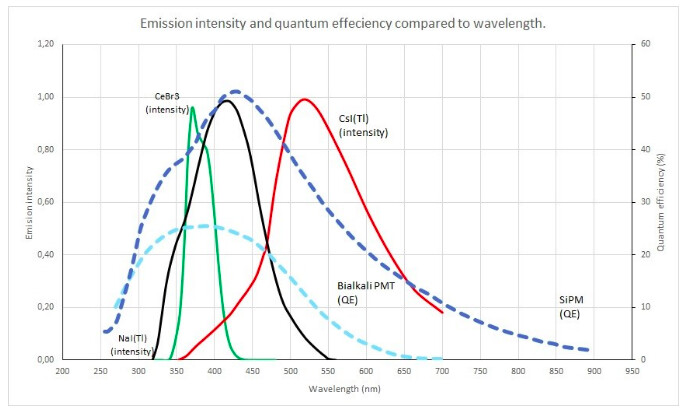
Okay, I understand.
I just have a small doubt , I have this emission spectrum from the scionix crystals page, the one you passed me is also quite useful and what I should do is to place the values of the emission intensity with their respective wavelength , an example according to the image I put would be that at 350nm the intensity is approximately 0.40 and thus fill the matrix FASTCOMPONENT and SLOWCOMPONENT with the entire spectrum.
Thank you very much for your help.
You must first convert all wavelengths to energy (in eV) according to Formula E=hc/λ and then place them in the NaI_energy array as shown below (The numbers used are not real):
G4MaterialPropertiesTable * NaI_mat= new G4MaterialPropertiesTable();
G4double NaI_energy[3]={1eV,2eV,3eV};
G4double NaI_SCINT[3]={0.5,1,0.4};
NaI_mat -> AddProperty ("FASTCOMPONENT", NaI_energy, NaI_SCINT, 3)->SetSpline(true);
Note: The NaI_SCINT array is the Emission Intensity in the emission spectrum that corresponds to each wavelength (energy). You can use [https://refractiveindex.info] to convert wavelengths to energy
abedbayat thank you very much for your help, it was very useful, now I know how to fill out the FASTCOMPONENT matrix, best regards
And where to find YIELDRATIO?
Also how to convert channel# to energy?
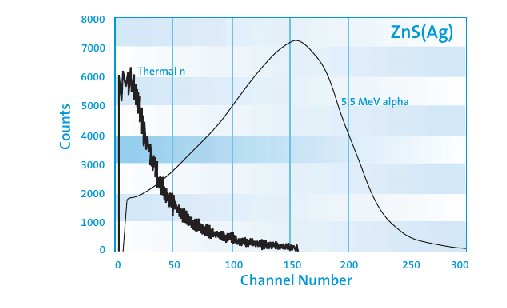
Hello,
To find the YIELDRATIO, you can use related articles. ZnS-Ag6LiF-Jlumin.pdf (376.2 KB)
See Table 1 in the article. A1, A2, A3 Equivalent to YIELDRATIO
YIELDRATIO needed when the scintillation level excitation is different for different types of particles.
The wavelength spectrum is also given in this article and you dont need to convert the channel into energy. Here the wavelength must be converted to energy (E=hc/λ)
Literally, ZnS:Ag (sensitive to alpha particles) has different scintillation properties than ZnS:Ag/6LiF (mainly used for neutron detection) but references in the paper was useful.

